R&D and Product Overview
We specialize in the development of high-potential innovative biopharmaceutical solutions of non-monoclonal antibodies and specific dosage forms that require an advanced level of technological capabilities.
Non-monoclonal Antibody Drugs
Our middle- and long-term biological pipelines which include biosimilars and biobetters worth billions of US dollars in current market value.
- Biosimilars
The efficacy and safety of our biosimilars have been proven in the market, which has led to high demand and sales. The development of biosimilars is less risky and can help us generate revenue comparatively quickly, which balances out the risks associated with the development of new drugs.
- Biobetters
We have successfully developed many innovative long-acting protein-based drug products by applying our patented platform for biobetters. These products have the advantages of long half-lives and excellent biological activity. They are also expected to achieve therapeutic effects at lower doses, further reducing their immunogenicity risk and increasing patients’ safety, thus providing patients with safer medication options.
- UB-851 Recombinant Human Erythropoietin (EPO)
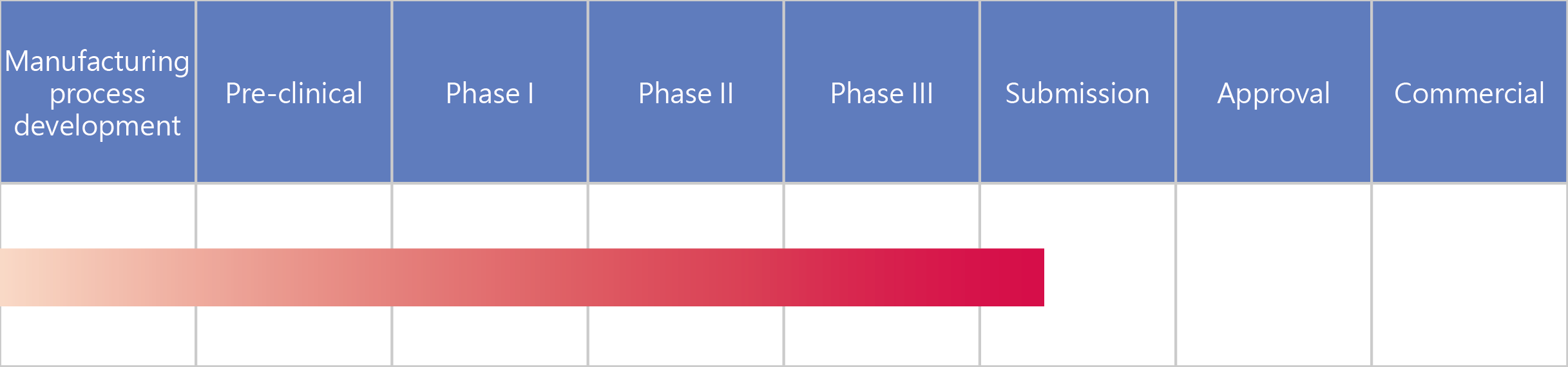
UB-851 Erythropoietin (EPO)
Erythropoietin (EPO) is a glycoprotein cytokine produced by interstitial fibroblasts in the renal cortex. It regulates the production of red blood cells in the body and promotes the differentiation of red blood cell precursors in the bone marrow into red blood cells.
Recombinant human erythropoietin is synthesized using cell cultures that have undergone genetic engineering. UB-851 was developed based on its brand name drug following the guidelines of the European Pharmacopoeia. It has demonstrated a high similarity to the reference drug in terms of its protein structure and efficacy in animal studies and human clinical trials. Furthermore, UB-851 has also passed strict quality control inspections and analyses.
- UB-852 Innovative long-acting EPO
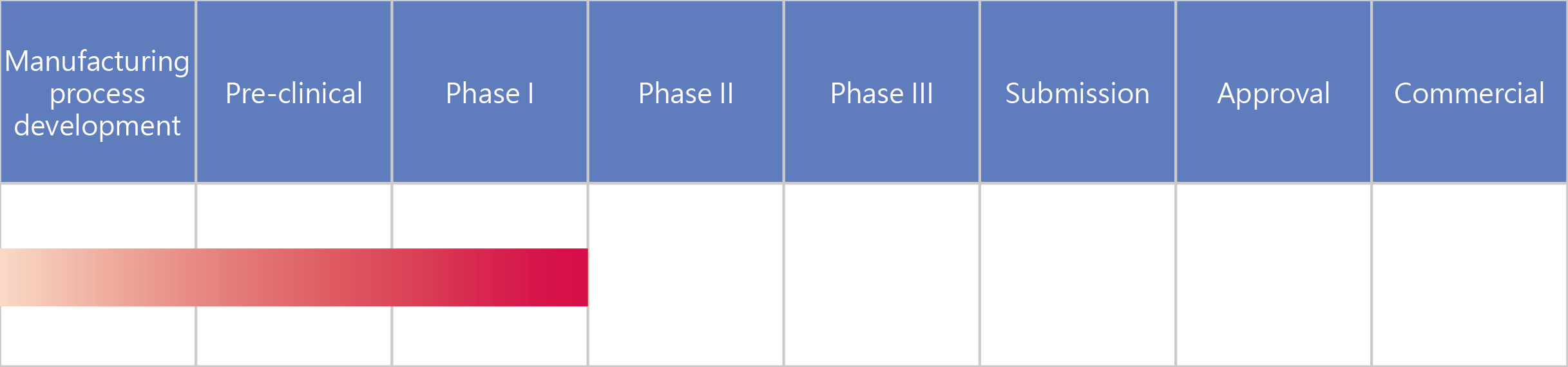
UB-852 Innovative long-acting EPO
EPO can be used as a long-term treatment for patients with renal anemia.UB-852 is a specially-improved EPO with an extended half-life, which improves patients’ willingness to take their medicine and enhances their quality of life.
We designed UB-852, a novel long-acting EPO, using UBI Pharma’s Patented Polysaccharide-binding Fusion Protein Platform, fusing natural erythropoietin sequences to proteoglycans. We can ensure the safety of UB-852 and eliminate concerns regarding the buildup of chemical substances in the body.
- UB-854 Recombinant Factor IX
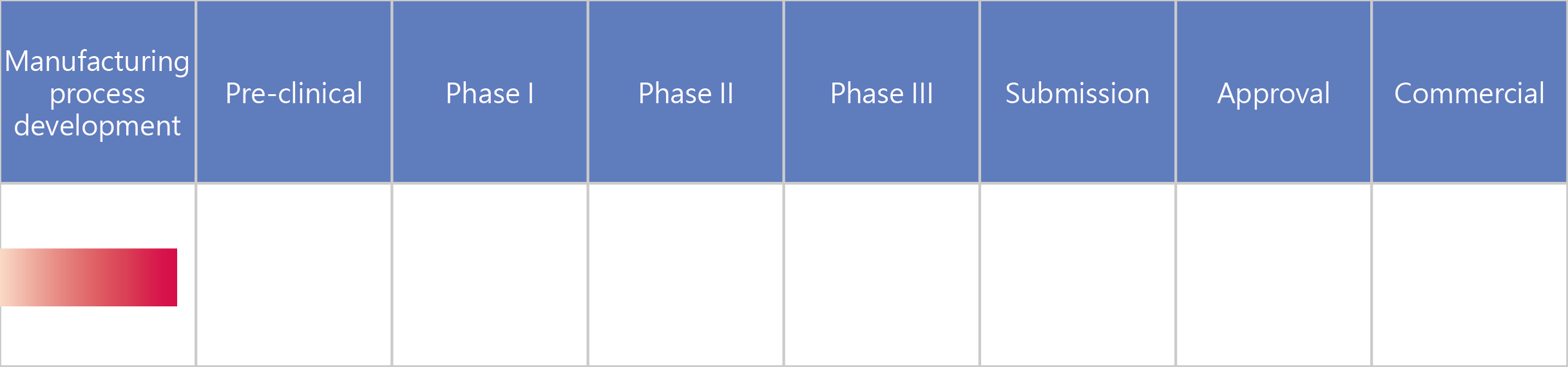
UB-854 Recombinant Factor IX
We applied genetic engineering technology to develop UB-854 based on high-producing mammalian cell lines for protein expression and strict screening methods. For production, continuous perfusion cultures were adopted. UB-854 is one of the few successfully developed recombinant coagulation factor IX products in the world, and boasts unique advantages in terms of its manufacturing processes and quality, which conform to the European Pharmacopoeia standards.
- UB-551 Innovative long-acting IFNα8
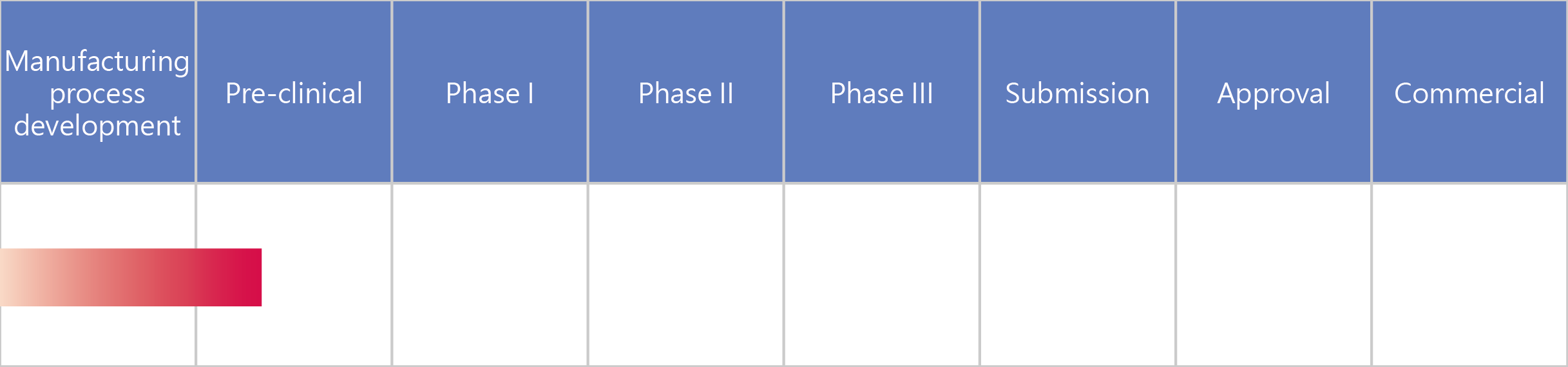
UB-551 Innovative long-acting IFNα8
UB-551 was designed using UBI Pharma’s unique fusion protein patented technology, which greatly extends its in vivo half-life. This drug provides several advantageous features, including high biological activity, low dosage, excellent safety, and easy industrial-scale production.
In addition to treating diseases for which general interferons are indicated, such as hepatitis B and hepatitis C, UB-551 also offers superior therapeutic effects in cancers such as breast cancer, prostate cancer, ovarian cancer, malignant melanoma, and colorectal cancer. It can be used alone, or it can be used in combination with other anti-cancer drugs to enhance the anti-tumor effects.
- UB-853 Innovative long-acting GCSF
UB-853 Innovative long-acting GCSF
G-CSF, which can stimulate the growth of myeloid cells, is used clinically in cancer patients with chemotherapy-induced neutropenia to minimize infections. It can also be used to obtain peripheral blood stem cells from donors by mobilizing blood stem cells into peripheral blood for collection.
The innovative long-acting GCSF UB-853 was designed using UBI Pharma’s unique fusion protein technology. It features advantages such as a long half-life, high level of safety, great biological activity, and good manufacturability.
UBI Pharma Inc.
No.45, Guangfu N. Rd., Hukou Township, Hsinchu County 303036, Taiwan (R.O.C.)
- Tel:+886-3-5977676
- Fax:+886-3-5981173





