Professional Services
We offer a wide range of dosage form manufacturing and development capabilities, providing high pharmaceutical quality that has been broadly certified.
We own the first U.S. FDA-approved manufacturing facility for injections in Asia (besides Japan).
Facility & Capacity
- Top Pharma Outsourcing Company in APAC 2002, awarded by Pharma Tech Outlook.
- Committed to excellence, reliability, and customer satisfaction.
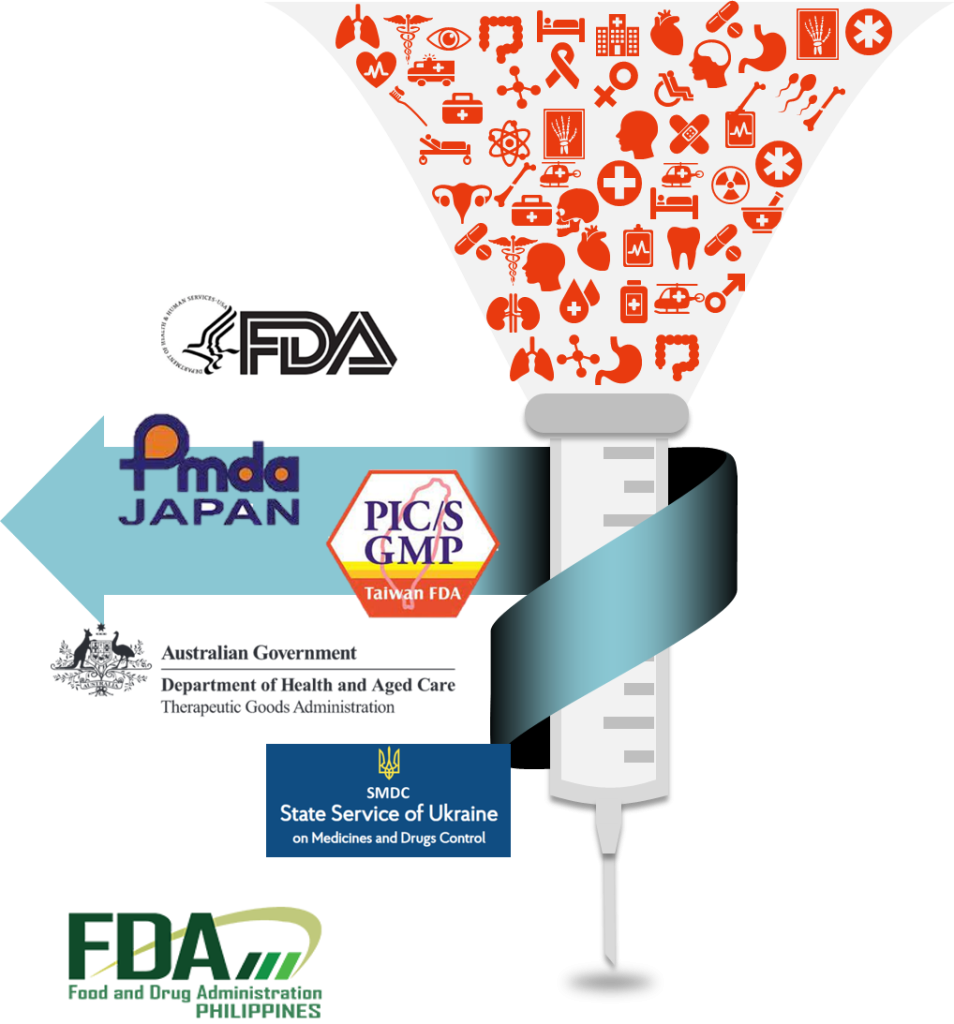
UBI Pharma Inc. upholds the legacy of GSK’s (GlaxoSmithKline) pharmaceutical plant, which complies with the European Union’s Good Manufacturing Practice (GMP) standards. Building upon this strong foundation, we remain steadfast in our commitment to GMP principles, ensuring exceptional product quality. Throughout the past two decades, our track record stands strong without a single product recall incident.
At UBI Pharma Inc., we cherish every customer, every order, and every aspect of our operational processes. We continuously strive to enhance our quality system to meet international standards. As a result, we have garnered significant achievements in quality certifications worldwide, establishing ourselves as one of the foremost injectable manufacturers in Asia.

- Never Compromising on Quality • Trusted Worldwide
At UBI Pharma Inc., we prioritize pharmaceutical quality as the cornerstone that sets us apart. Our unwavering commitment to uncompromising quality is the driving force behind our operations.
Located in Hsinchu, Taiwan, our facility has undergone rigorous inspections by multinational regulatory agencies, including the US FDA. We are proud to share that throughout the past decades, we have maintained an impeccable record with no instances of product recalls, despite offering a diverse range of pharmaceutical products.
With this solid foundation, UBI Pharma Inc. stands as the ideal partner you have been seeking. Our pride and confidence stem from our exceptional quality system, ensuring that we consistently deliver the best solutions in the industry.
- Quality Policy
- Ensuring that every product used by our customers is manufactured according to our high level of quality standards and will be safe and effective.
- To take the lead in raising the quality of pharmaceutical production in Taiwan in every aspect, including production, manufacturing, and marketing, with the ultimate goal of improving human health.
- Diversified Production Lines
- With a state-of-the-art facility spanning 240,000 square feet, our diverse product portfolio spans a range of non-sterile offerings, including tablets, capsules, sachet, oral solution, and topical ointment to sterile products like vials, ampoules, and freeze-dried preparations.
- At UBI Pharma Inc., we continuously strive to lead at the international level in terms of service and technology. With a strong foundation in professional technology and a customer-centric approach, we are dedicated to provide our clients with flawless quality and exceptional service, thus becoming their most reliable and trusted partner.

- State-of-the-art Equipment
- Non-sterile
- Unipress Compressor
- Drum Blender
- Aeromatic FBD
- Filder Granulator
- Metal Detector
- Gemcomatic Blender
- Capsule Filling Machine
- Super Coater YC-EP-SC 125F+S
- Tabletting Machine
- Metal Detector
IWK Tube Filling
Becomix Homogenizing Mixer
1000L Manufacture Vessel
- Sterile
- Headspace O2 Analysis
- Ampoule Filling Machine
- Autoclave
- Oven
- Automatic Inspection Machine
- Vial Washer
- Hot Air Sterilizing Tunnel
- Vial Filling and Closing Machine
- Autoclave
- Autoclave+VHP
- Vial Washing Mahcine
- Heat Tunnel
- Filling Machine
- Capping Machine
- 500L CIP/SIP Compounding System
- H2O2 Fumigation Machine
- Filter integrity tester
- Inspection Machine
- Lyophilizer
- Optrel Inspection Machine
- Kaye Validator
- Filter integrity test device
- IWK Tube Filling
- Becomix Homogenizing Mixer
- 1000L Manufacture Vessel
- Packaging
- Tablet Counting Machine
- CAM PMM Cartoning Machine
- CAM AV Cartoning Machine
- Tablet Counting Line
- Sachet packing machine
- Blister Filling Machine
- Cartoning Machine




UBI Pharma Inc.
No.45, Guangfu N. Rd., Hukou Township, Hsinchu County 303036, Taiwan (R.O.C.)
- Tel:+886-3-5977676
- Fax:+886-3-5981173





